Canopy Cancer Collective brings together medical care providers, interdisciplinary teams, cancer researchers, and philanthropic and industry partners in an effort to develop and scale best practices and innovations applicable to the fight against pancreatic cancer. No content on this site is to be considered to be a recommendation or medical advice.
Resources
-

White Paper
From Overlooked to Essential: Transforming Pancreatic Cancer Care with EPI Management
The First U.S. Consensus Statements on Identifying and Managing Exocrine Pancreatic Insufficiency in Pancreatic Oncology Practice -
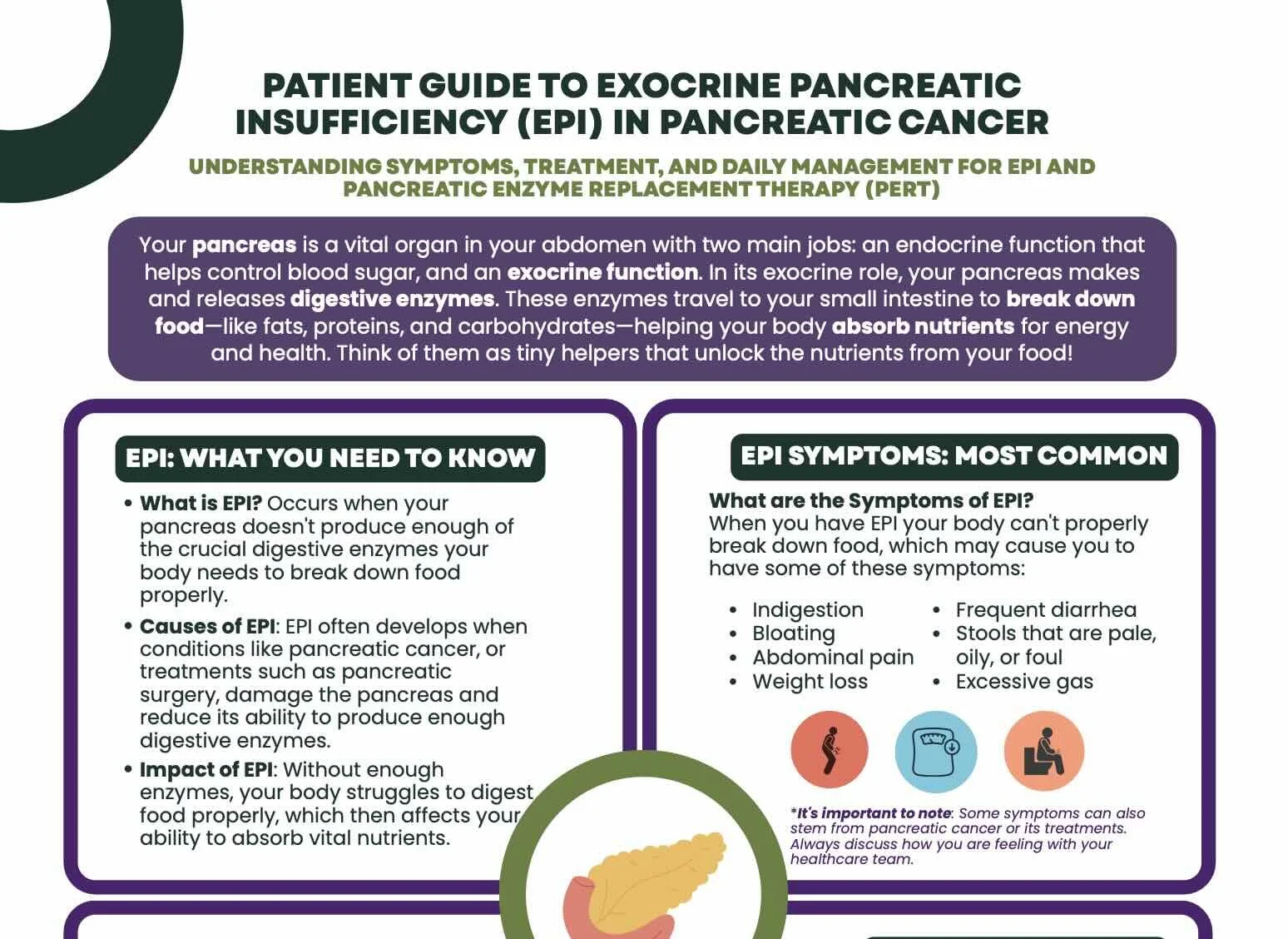
Patient Guide
Patient Guide to Exocrine Pancreatic Insufficiency (EPI) in Pancreatic Cancer
Understanding Symptoms, Treatment, and Daily Management for EPI and Pancreatic Enzyme Replacement Therapy (PERT)
-

Tool
A Summary For Healthcare Teams:
The First Consensus Statements on Identifying and Managing EPI in Pancreatic Oncology in the U.S.
Under no circumstances does Canopy provide any medical advice and it is not licensed to do so. By accessing and using this site, you acknowledge that there is no professional relationship, including but not limited to a doctor-patient relationship, between you and Canopy or any of its employees, professionals, partners, agents, creators, or authors (collectively “Affiliates”), and neither Canopy nor any of its Affiliates shall have any liability whatsoever in that regard. Canopy and its Affiliates specifically disclaim any and all liability for personal injury, property damage, or other damages of any kind, whether special, indirect, consequential, or compensatory, that may result directly or indirectly from the use of, or reliance on, any publication or content on this site.
No part of any publication or content on this site may be reproduced, distributed, or transmitted in any form or by any means, including photocopying, recording, or other electronic or mechanical methods, without the prior written permission of Canopy, except in the case of brief quotations for non-commercial, educational, or critical review purposes, as permitted by copyright law. For permission requests, please contact Canopy Cancer Collective at support@canopycancer.org. Canopy provides publications and content as a service to its members and community, which are for informational purposes only.
